This blog highlights research and development of new treatments, outcomes of global clinical trials and their regulations, with a special focus on ophthalmology and retinal diseases, including drugs and devices. The blog is meant for both clinical and pharmaceutical experts, as well as for patients / family members who are looking for information.
Wednesday, November 15, 2017
FDA approves the first digital pill that tracks compliance
 |
| Source: Proteus Digital Health |
Tuesday, November 14, 2017
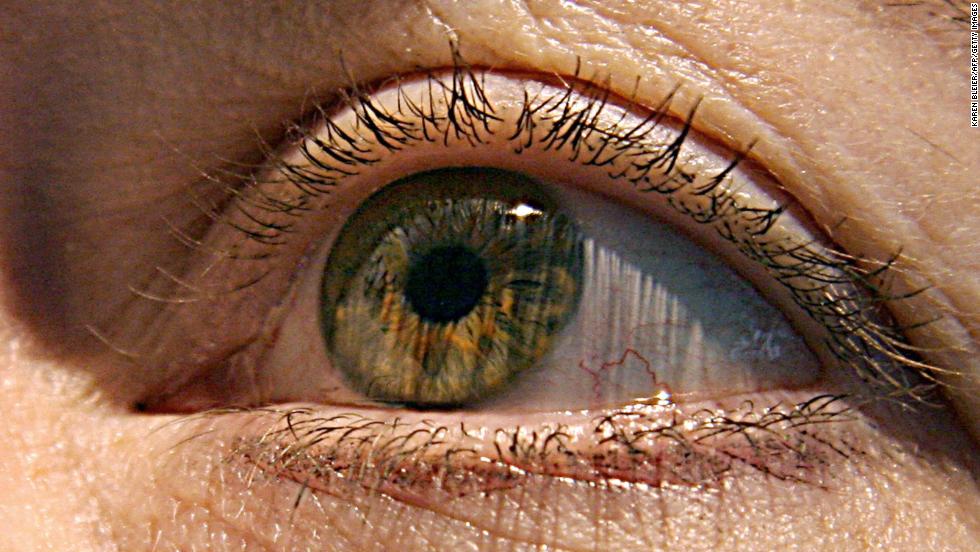
Studies show stem cell-based treatment safe in macular degeneration
Results from two early clinical trials show that it may be possible to use human embryonic stem cells as treatment for the dry form of macular degeneration, according to presentations at the American Academy of Ophthalmology Annual Meeting 2017 in New Orleans, LA. Stem cells injected into the eye appear to have replaced the missing cells damaged by the disease, with no serious side effects.
Saturday, November 4, 2017
Does performing/undergoing surgery in the afternoon reduce the risks?
 |
| (c) Genetic Literacy Project |
A new study suggests patients undergoing open heart surgery in the afternoon have a lower risk of potentially fatal complications than those undergoing operations in the morning. The study found that events including heart attacks and heart failure were less common among those who had undergone a valve replacement operation in the afternoon. The finding appears to be linked to the ability of the heart tissue to recover after being starved of blood supply during surgery – an effect the researchers say is influenced by the cells’ biological or “circadian” clock.
Friday, November 3, 2017
The first dual-action Glaucoma drug, Vyzulta, approved by FDA
 |
| (c) AAO |
Orion, the Cortical Visual Prosthesis System, gets full FDA approval
 |
| (c) medgadget.com |
Subscribe to:
Posts (Atom)