 |
| (c) NEI |
Please read the disclaimer here.
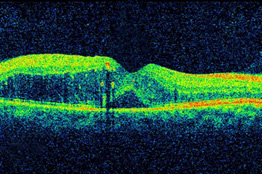
The TYBEE trial (NCT03126786) is a multicenter, randomized, masked, controlled Phase 2 trial, which has enrolled 71 treatment-naïve DME subjects for this study of 6 months duration.
In the trial, subjects were randomized into:
- Combination arm received suprachoroidal CLS-TA [40 mg/mL] together with intravitreal Eylea [2 mg (0.05mL)]
- Control arm received intravitreal Eylea alone (with sham suprachoroidal injection)
The primary outcome measure is a comparison of mean change from baseline in best corrected visual acuity between the two study arms. An additional analysis will be a comparison between the number of injections required between the two groups.
 |
| (c) Retinal Physician |
Fluid rapidly diffuses into the posterior segment following administration through the SCS in animal models; up to 1 mL of fluid is accommodated in the space. This volume is larger than what is required for achieving therapeutic levels that are clinically relevant for drugs. Injections of 10 to 50 µL into the SCS are well tolerated with a low risk of ocular complications.
Fluid injected via the SCS spreads around the globe both on top of and through the choroid, distributing through the choroid and the retina. Should a drug be injected, the drug solution or suspension spreads in a similar manner, accessing the choroid and the retina. In contrast, when the same drug is injected into the vitreous, the drug spreads diffusely across all parts of the eye. 2
In animal studies performed previously, steroid Triamcinolone acetonide (TA) was detectable throughout the 91 day period in eyes after dosed by suprachoroidal administration. Based on area under the curve analysis, 96% of the drug was exposed to SCR and retina after SCS administration. Exposure to TA was very low in the anterior chamber including: aqueous humor, iris, lens, and vitreous humor following suprachoroidal injection. Also, suprachoroidally injected CLS011A showed drug levels in the SCR that were at least 3 orders of magnitude higher than vitreous and drug was below limits of detection (1 ng/mL) in the aqueous.By maximizing drug levels in retina, while minimizing levels in anterior part of the eye, the potential to develop cataract and increased intraocular pressure is reduced. Cataract and glaucoma have been a problem in relation to long term use of steroids in conditions such as DME (not amenable to anti-VEGFs) and uveitis, where repeat or sustained-release steroids have lead to the above complications.
A significant number of patients with DME (~40-50%) do not respond well to anti-VEGFs alone. Referred to as non-responders or refractory, these patients tend to worsen in spite of repeat treatments. Considering the progressive nature of the disease, retinal specialists have been using steroids in various formulations, including intravitreal injections and sustained release products. Though the latter have shown good outcomes in refractory patients, the issue with cataract and glaucoma continues to cause concerns for the treating physician and for the patient.
We await results from this study to see whether suprachroidal injection of TA is able to control the disease process in these subjects with DME. Also, as an add-on trial, it might help to evaluate outcomes with the combination therapy (eylea+TA) in refractory DME patients alone, which currently do not have much recourse to treatment.
No comments:
Post a Comment